April 16, 2024
By Savanna Newkirk and Lily Flaherty
As the semester progressed, we continued to have numerous opportunities to improve our lab skills and gain more experience. Throughout the last couple of weeks, we have worked on guided experiments and have also created two of our own. In our last blog post, we discussed the inner workings of the beginning of our seed germination experiment. As time has passed, we have had multiple seeds germinate, meaning that we could start planting them. The first to germinate were the controlled spider milkweed (Asclepias viridis), followed by the boiled A. viridis. The wild hyacinth (Camassia scilloides) species was slower to germinate, but there were signs of germination roughly two weeks after the first A. viridis began germinating. Unfortunately, most of our sprouts died within days of being planted. In the coming final weeks of the semester, we will be analyzing our results using statistical methods.

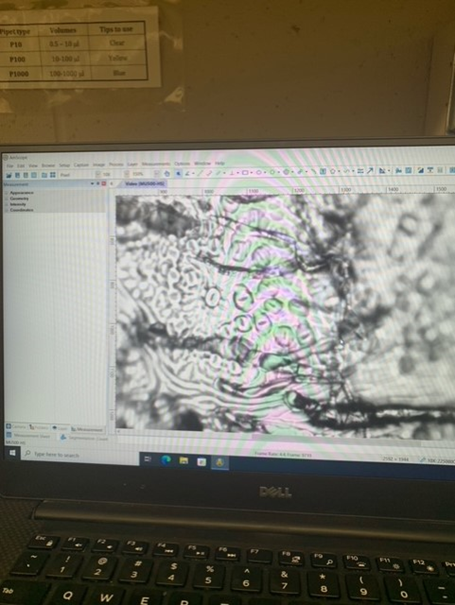
Since our last post, we also designed and conducted an experiment that focused on the impact that drought conditions have on stomata. Our experiment involved two species of Oak, Quercus laevis and Quercus virginiana. For each species, we had two plants that we used, meaning four plants total. For each species, one plant was under drought conditions, while the other was watered well. We picked three leaves from each plant (twelve leaves total) and made stomata peels from each. We did this by painting a small strip of nail polish on the underside of each leaf, letting it dry, and gently peeling it off. We then put each peel on a slide and observed them under the microscope. We used the AmScope software to count the stomata and measure the aperture length and width. We measured the length and width of three stomata from each slide made. We have begun to analyze our results. We used a calibration slide to create a scale of pixels to millimeters, then used this scale to convert all of our data from pixels to millimeters. We also found the SPI (Stomatal Pore Index) of each slide that was made. We will continue to analyze our results by using Microsoft Excel or by coding in R.
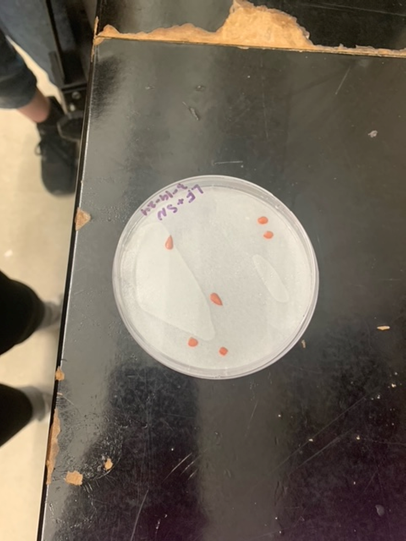
We also got the opportunity to conduct a Tetrazolium test (TZ test) on cup plant (Silphium perfoliatum) seeds. A TZ test has the ability to test seeds for viability in a short time frame. To conduct the TZ test, we first acquired three cup plant seeds that had been soaking in water in order to release their hydrogen ions. Following this, we cut the seeds in half lengthwise and placed them cut side down in a petri dish with a TZ solution-soaked filter paper. The seeds were then left to sit at room temperature for 2 days. The TZ solution reacts with the hydrogen ions, staining the live tissue in the seed red while the dead tissue stays white. In all of our seeds we found that the embryo of the seed was stained red, meaning that all three of our seeds were viable.