By: Reika Hallett and Zach Leaf
1 October 2021
After completing the downstream region of our gene deletion plasmid, pZR01, derived from Flavobacterium psychrophilum, we have started to perform the same steps to clone the upstream region of our gene just like we did with our downstream DNA. Since the start of the semester, we have primarily been focusing on trying to ligate and transform our upstream region into our plasmid, but as enjoyably intriguing the research has been going so far, we have been running into quite a few issues and troubleshooting along the way.
Just like we have performed with our downstream region, we have run a Phusion PCR to amplify the upstream DNA and run a gel of our PCR product. The images from our gel came out great, and after having successful results, we then observed the concentration and purity of our samples using a nano-drop which showed that our concentration was exceptionally high, and our purity was decent. Afterwards, we purified our Phusion PCR product and digested it alongside our plasmid (PZR01) using restriction enzymes. We need to cut out of our plasmid as well as the PCR product we must ligate or glue back into that plasmid.
After we purified the digested PCR product and plasmid we then took a break before we performed ligation in order to practice our skills by doing a gel extraction. Usually, you perform a gel extraction when you run a gel electrophoresis and your samples don’t turn out super pure. To perform this experiment, we set up a gel electrophoresis and just used the 1kb DNA ladder as our sample. Once we ran the gel, we cut out two bands to perform the extraction, the .5kb band and 3kb band. After we cut these bands, we incubated them in a buffer with a high temperature so they would melt. Then we performed purification on the melted gel and then set them aside to run a gel with the digested plasmid and Phusion PCR samples.
After we ran these four samples, we realized something must have gone horribly wrong because only one band showed up out of the four and it was our plasmid sample. But then we realized that the band that showed up was at 2kb and our plasmid sample was at least 8kb big so we thought that there must have been an error with our gel. So, we decided that we should just run another gel with the same four samples just to see if it was just a fluke. So, we ran a second gel, but the results were the exact same.
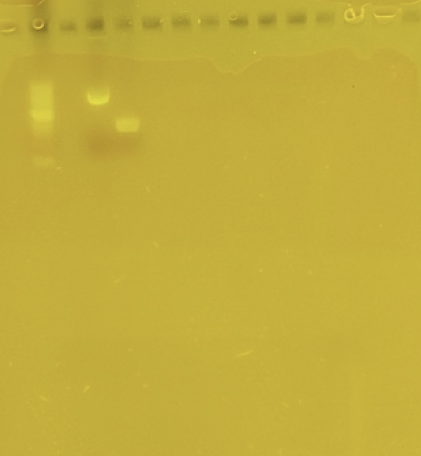
Due to our “plasmid” showing up as 2kb and our PCR product not showing up at all even after our second attempt on our gel electrophoresis, we have created another Phusion PCR. Also, we have found out that the plasmid from our previous gel wasn’t correct; we accidentally loaded a fragment that was only 2kb instead of our 8kb plasmid. Knowing our mistake, we purified our new Phusion PCR and ran a gel on that alongside our actual plasmid: the outcome was relieving to say the least.\
In conclusion, our start to this semester of work has been a little rocky with a few setbacks here and there. But now we are back on track. We just finished doing a gel with our new Phusion PCR and correct Plasmid as well as purification of our new PCR. Next, we will be doing a digestion of these two samples followed by ligation.


