Introduction:
From our last blog post, we were having difficulties with our plasmids and having good concentrations to move forward in our research. To say the least, we have not moved very far from that point. We have continued having difficulty getting good concentrations and sequencing results. We did do a new protocol to help us with different techniques when it comes to research, and we have finally started working on our posters for our presentations within the next couple of weeks.
Specific content:
Since our last blog post, we have not completed much more research in terms of the samples we have been working with throughout the entire semester. We have continued with the same protocols in the hopes of receiving better results for sequencing. The protocols have included ligation and transformation, restriction digest, and sequencing our plasmids.

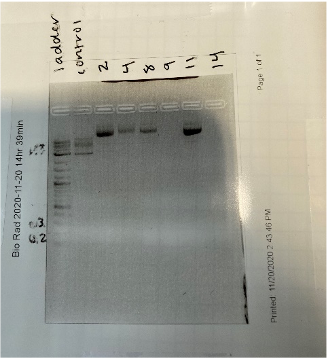
Within the past couple of weeks, we have scraped one of our samples solely because we continued receiving poor results or low concentrations when it came to the nanodrop. With that in mind, we moved forward with two out of our three samples to conduct our research. However, we kept seeing poor results, so we ended up starting over with growing up colonies in a broth which we then allowed to grow overnight. Thankfully, our samples did have growth, so our group was able to push forward with these two samples as we had in the past. With those samples, about 13 out of the 16 that grew had decent concentrations to work with. We only chose three per sample to run in a gel after restriction digest. When we ran the gel, it did not come out as we had imagined. The gel looked smeared, the bands were too high up when compared to the DNA ladder that we included in our gel, and there were no faint bands at the very end of our get to show proof of our plasmid taking up the insert as we had expected. With that in mind, we still sent photos of the gel off to Dr. Land to see if we could still have our samples sent off for sequencing.

Apart from our usual routine of ligation and transformation and a restriction digest, we went up to the cell culture lab for two sections with our lab TA Courtney. While we were there, we were able to distance ourselves from the routine we had in place for what felt like months. We worked with HeLa cells which we have not worked with since the very beginning of our research last semester. With Courtney’s help, we began incubating the cells to practice counting the amount of live and dead cells within a 4×4 grid. We let our samples sit overnight and incubate before we came back the next day to work hands on with pipetting and working under the hood ourselves. We all had a really good time with the cell culture lab overall.
On top of everything else, we began working on our posters for presentations on December 1, 2020. We have been hard at work ensuring everything is to our standards and laid out nicely to allow our presentation to go smoothly.
Conclusion/Reflection:
Overall, we are quite upset our samples have not worked the way we wanted them to. We were aiming to at least get to gel purification, but our samples seemed to have struggled this semester. Nonetheless, we did not let that put us down in our research. We continued to strive to get the best results we could every week. Our samples did not work, but we were still able to practice being in the cell culture lab for the future if we continue to go on with research. As a group, we are happy to have been able to work together this semester. We learned a lot from trial and error, especially the error side. Even though this is the end of our research this semester, we are looking forward to the future when we can continue with research.

