November 17, 2019
By Amyah Ockenga and Platung Chang
Introduction:
As we are moving forward with our research in fall of 2019, our goal remains the same: to determine miRNA regulations of APOBEC3A and/or APOBEC3B genes, which are found to be in cancers. With our progress from rescuing our plasmids, we have been able to test the samples hsa-miRNA-3135b and hsa-miRNA-374c-5p through ligation and transformation, and screening. As of today, we have successfully cloned our miRNA into the pcDNA 3.1 vector, which was shown through sequence analysis. This succession has led us to move forward with our research and proceed to our next phase which will be to plate and transfect the pcDNA 3.1 miRNA and psiCheck2-A3-3’UTR into HeLa cells.
Research:
Proceeding with our success so far, we have started by plating and transfecting mammalian cells in culture. Before actually using our miRNA samples, we wanted to practice plating, counting, and transfecting cells in culture because this was a very new protocol for us. We first wanted to count the number of live cells in the DNA sample, but to do so we needed to plate them first. To plate them we warmed media trypsin to 37 degree Celsius, then removed the media in the cell plate and rinsed it with PBS-EDTA which is phosphate buffered saline EDTA. With the clean plate of cells, trypsin was added, and the cell plate is then transferred to the incubator set at 37 degree Celsius for about 30 min.
To count the cells, we used a “hemacytometer” which is a tool that helps you count using chambers, which each have a tiny grid engraved into them. Using a pipette with 10 ul of the cells, we placed them into the groove of the hemacytometer. This allowed them to flow under the coverslip that was placed on top. We then examined and counted our cells under a microscope. Using the four x four grid to count our cells, we had 17 cells in the first square, 19 in the second, 30 in the third, and 20 in the fourth.
Future Steps:
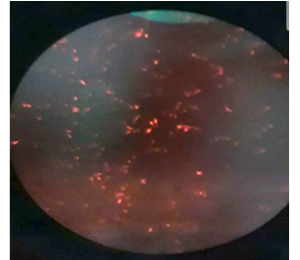
Next was transfecting, to transfect we wanted to pass along a gene on a plasmid to the nucleus of a cell where it could be expressed. We conducted this by aliquoting 10 ul of DNA into transfected tubes. We then calculated a master mix of 8 ul of PEI and 200 ul of SFM (Serum- Free Media), which is used for each well of a 6-well plate. Because we only plated one well, we only needed that amount of each component. After aliquoting the master mix to the DNA tube, we incubated it for 30 min and then added it to the cells. These cells were then placed back into the incubator. After two days, we observed our transfected samples under a microscope and the bright fluorescence red is the result of a successful transfection. After practicing, we started plating and transfecting our own miRNA 374c-5p with APOBEC3B gene.
After transfection, we will move to Dual Luciferase Assay using our miRNA. This will assess the ability of our miRNA to silence the APOBEC3A/APOBEC3B gene. While conducting Dual Luciferase Assay, we will also be trouble shooting our second sample hsa-miRNA-3135 to determine whether it will be able to ligate and transform successfully.

